What is Wet Conditioning?
Wet conditioning is conducted before the fluid bed dryer (FBD) process, whereby the wet granulate is passed through a mill in order to provide a uniform granule for drying. After the FBD the dried material is milled again to achieve the particle size as stipulated for the manufacturing process.
Milling Wet Conditioned Powders
The manufacturer’s experiment[1] involved testing two different processes side-by-side to examine and compare the outcomes of wet conditioning. In both cases, wet granulate was used as the starting point, with two different levels of solution being added - high and low amounts of solution.
For process A, the wet granulate was wet milled, dried in the fluid bed dryer, and then the dried powder was milled again.
For process B, granulate was sent straight to the fluid bed dryer and once dried was milled using the same mill set up as in process A.
In both experiment streams, a Quadro Comil was used for the dry milling step. For dry milling, the Comil was fitted with one of three screen sizes: a 1 mm round hole screen (039R), a 1 mm grater screen (040G) and a 1.8 mm round screen (075R) to compare particle sizes based on hole size and hole profile. All tests utilized a round bar impeller (1601) running at various RPMs (to further compare effects of RPM on PSDs). In Process A the wet granulate was milled using a Quadro Comil® fitted with a 9.5 mm square-hole screen (375Q), and a square bar impeller (1607) running at 2000 RPM.
The results of Wet Mass Conditioning
Process: A wet mass conditioning produced similar-sized granules which were then sent into the fluid bed dryer (FBD). Because the particles were of the same size they dried at the same rates. This avoided the creation of moist centers, which can occur in larger particle agglomerations, where the outside dries quickly enough, but the center remains wet. This causes problems at the dry milling stage (after the FBD) where they will smear on the screen (see picture below).
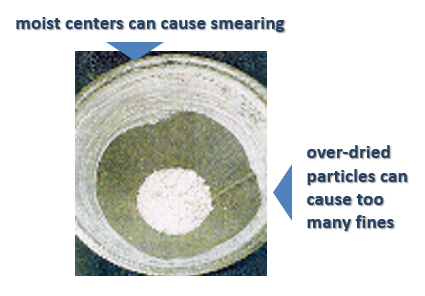
In addition, without sizing wet granules successfully there will also be small particles created which will become over-dried in the FBD. These then become overly hard and difficult to break down in the milling process, they have too long residence time in the mill which increases the amount of recirculation and causes fines. This production of fines compromises the quality of the product as these particles may have a different percentage of active ingredient, which will affect the ratio of the active ingredient in the final tablet. Furthermore, the creation of fines affects powder flowability to the tablet press and tablet compaction. A high percentage of fines could affect the quality of the tablets resulting in dosage, weight and robustness variations, as well as compromised dissolution rates, efficacy and bioavailability.
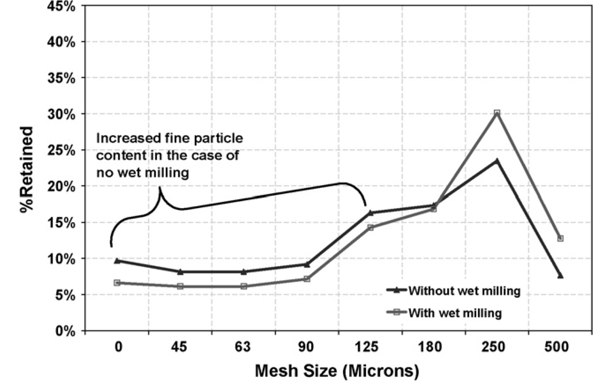
Proof of reducing the amount of fines
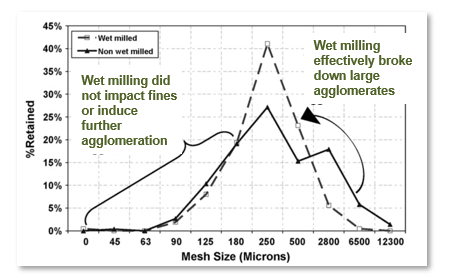
The chart below shows the particle size distributions (PSD) of the wet material produced by the two processes. Process A, where the Comil was used to de-lump the wet mass (dotted line) and Process B (solid line) where no wet milling step was used. The wet-milled material produces a much tighter PSD of wet granules, yet does not impact the production of finer granules at the lower end. In other words, it made the distribution of the wet mass entering the FBD more uniform.
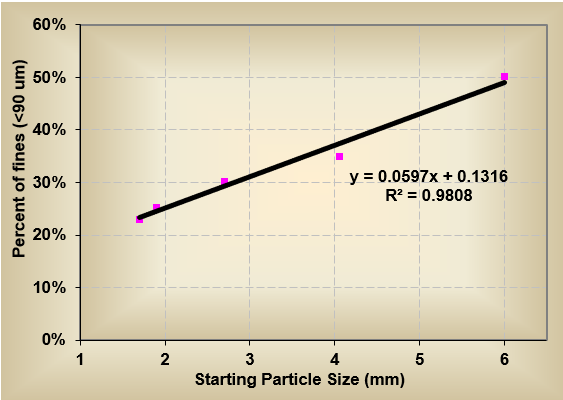
In addition, they discovered that the smaller the diameter of the granules entering the drying stage, the more uniform the milling results (chart below). Reducing the granules to 2mm before drying generated approximately 20% fines, whereas larger granules (6 mm) generated closer to 50% fines (fines were considered particles under 90 micron). This difference was attributed to overall residence time of the granules inside the mill. The smaller the granule entering the dry mill, the faster it could be size reduced. Larger granules had longer residence time inside the milling chamber leading to more particle-to-particle attrition and fines generated from over-circulation.
Therefore, when comparing the dry milling results between Process A and Process B (with and without the wet milling step prior to the drier), it was evident that the wet milling step resulted in an overall reduction of approximately 20% in the generation of fines (chart below).
The material properties
There had been a concern at the outset that squeezing the wet mass through a screen would densify the material, but this was proven not to be the case. In fact, the wet conditioning process did not negatively impact on granule porosity, drug assay distribution or compatibility and produced a high quality of finished product.
Overall benefits of wet milling
The wet milling process (Process A) eliminated the larger agglomerates (larger than 6 mm) which enabled the manufacturer to reduce drying time by approximately 25%. This had several advantages, not only could they realize cost savings for the drying process, but decreasing the drying time lowers the thermal stress on the active ingredients, which also enhanced the quality of the end-product. In addition, because the quantity of fines was reduced by using the wet conditioning step, the flow properties of the material in the downstream equipment was enhanced and reduced the risk of size-based segregation.
In Conclusion
The experiments were deemed highly successful and the results enabled the manufacturer to change their process to include wet conditioning before the fluid bed dryer and improve product quality, product consistency, and efficiency.
If you’d like to see a demonstration of the Quadro Comil and perhaps do your own testing with wet granule conditioning, then please contact us and we’ll put you in touch with your local sales engineer.
[1] Reference – the original papers:
Investigation of Size Reduction Mechanisms in a Conical Screen Mill for Wet Granulation Before and After Drying. Presented at AAPS 2002, Toronto by Luke Schenck, Russell Plank, James Zega
Impact Milling of Pharmaceutical Agglomerates in the Wet and Dry States. Published in International Journal of Pharmaceuticals (2007) by Merck & Co. Inc., West Point PA Pharmaceutical Research and Design

